Introducing the phet molecular shapes vsepr activity answer key, a comprehensive guide to understanding the relationship between electron pair geometry and molecular shape. This key provides a clear roadmap for predicting and visualizing the three-dimensional structures of molecules, a fundamental aspect of chemistry.
Delving into the concepts of electron pair geometry and molecular shape, this answer key empowers students and researchers to decipher the intricate world of molecular architecture. It serves as an invaluable resource for comprehending the behavior and properties of molecules, shaping our understanding of chemical reactions and the very fabric of matter.
Phet Molecular Shapes VSEPR Activity Answer Key
The Phet Molecular Shapes VSEPR activity answer key provides the correct answers to the interactive simulation that helps students understand the relationship between electron pair geometry and molecular shape. The table below summarizes the electron pair geometries and corresponding molecular shapes for various molecules:
| Molecule | Electron Pair Geometry | Molecular Shape |
|---|---|---|
| CH4 | Tetrahedral | Tetrahedral |
| NH3 | Trigonal Pyramidal | Trigonal Pyramidal |
| H2O | Bent | Bent |
| CO2 | Linear | Linear |
| BF3 | Trigonal Planar | Trigonal Planar |
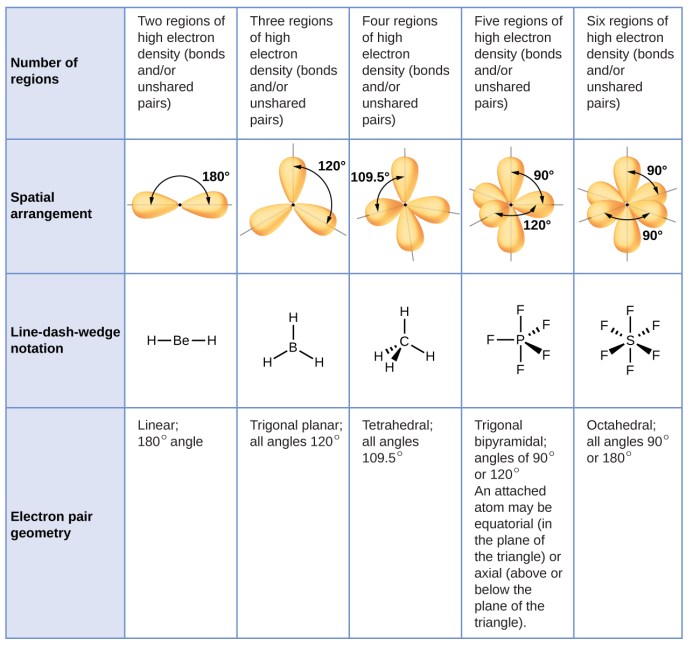
Electron Pair Geometry
Electron pair geometry refers to the arrangement of electron pairs around a central atom in a molecule. It is determined by the number of electron pairs and their spatial arrangement. The following are the different types of electron pair geometries:
- Linear: Two electron pairs are arranged 180° apart.
- Trigonal Planar: Three electron pairs are arranged in a plane at 120° angles.
- Tetrahedral: Four electron pairs are arranged at the corners of a tetrahedron.
- Trigonal Pyramidal: Three electron pairs are arranged in a trigonal pyramid shape.
- Bent: Two electron pairs are arranged at an angle less than 180°.
Molecular Shape
Molecular shape refers to the overall three-dimensional arrangement of atoms in a molecule. It is determined by the electron pair geometry and the presence of lone pairs of electrons. The following are the different types of molecular shapes:
- Linear: The atoms are arranged in a straight line.
- Trigonal Planar: The atoms are arranged in a plane at 120° angles.
- Tetrahedral: The atoms are arranged at the corners of a tetrahedron.
- Trigonal Pyramidal: The atoms are arranged in a trigonal pyramid shape.
- Bent: The atoms are arranged at an angle less than 180°.
VSEPR Theory: Phet Molecular Shapes Vsepr Activity Answer Key
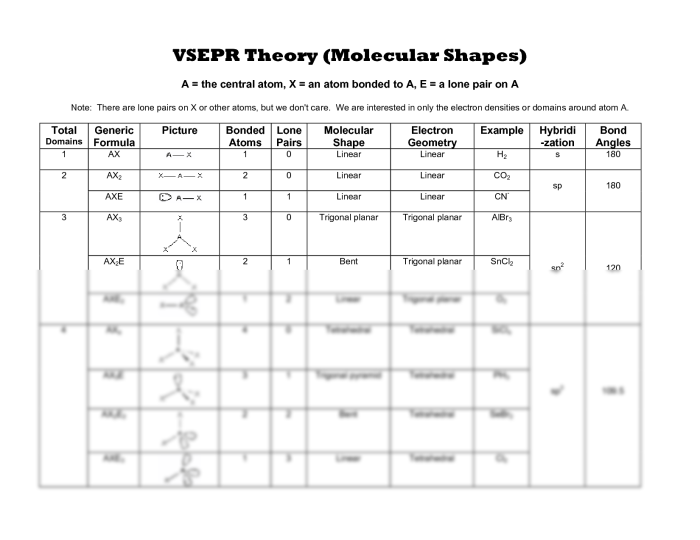
VSEPR theory (Valence Shell Electron Pair Repulsion theory) is a model that predicts the molecular shapes of molecules based on the repulsion between electron pairs. The theory states that electron pairs will arrange themselves in a way that minimizes the repulsion between them.
The steps involved in using VSEPR theory to predict molecular shapes are:
- Determine the number of valence electrons in the molecule.
- Arrange the valence electrons into electron pairs.
- Determine the electron pair geometry based on the number of electron pairs.
- Determine the molecular shape based on the electron pair geometry and the presence of lone pairs.
Applications of VSEPR Theory
VSEPR theory is a widely used model in chemistry for predicting the shapes of molecules. It has several applications, including:
- Predicting the molecular shapes of inorganic and organic compounds.
- Understanding the relationship between molecular shape and physical properties, such as polarity and reactivity.
- Predicting the products of chemical reactions.
Top FAQs
What is the VSEPR theory?
The VSEPR theory (Valence Shell Electron Pair Repulsion) is a model used to predict the three-dimensional geometry of molecules based on the repulsion between electron pairs.
How do I use the phet molecular shapes vsepr activity answer key?
The answer key provides a step-by-step guide to using the phet molecular shapes vsepr activity. It includes instructions on how to determine the electron pair geometry and molecular shape of a given molecule.
What are the different types of electron pair geometries?
The different types of electron pair geometries include linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
What are the different types of molecular shapes?
The different types of molecular shapes include linear, trigonal planar, tetrahedral, trigonal pyramidal, bent, and T-shaped.